immunoglobulin isotypes
The immunoglobulin superfamily comprises an evolutionarily ancient, widely expressed, constitutive or long-term up-regulated family of glycoproteins involved in cellular adhesion, signaling, and the adaptive immune response.
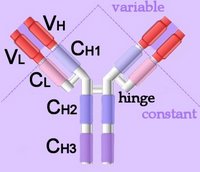 Immunoglobulins (left - click to enlarge) comprise two heavy (H) and two light-chain (L) protein subunits, each of which folds into domains (4 on heavy, 2 on light). These adhesion sites or domains contain one or more folds of 60 to 100 amino acids.
Immunoglobulins (left - click to enlarge) comprise two heavy (H) and two light-chain (L) protein subunits, each of which folds into domains (4 on heavy, 2 on light). These adhesion sites or domains contain one or more folds of 60 to 100 amino acids.
Depending upon the character of the heavy chain, immunoglobulins are divided into five classes – IgG, IgD, IgE, IgA, IgM – that are expressed in different tissues (detail). The classes are further subdivided into isotypes, which have different properties in terms of complement fixation and binding affinity to immunoglobulin (Ig) receptors.
Immunoglobulins can be divided into five different classes, based on differences in the amino acid sequences in the constant region of the heavy chains. All immunoglobulins within a given class will have very similar heavy chain constant regions. These differences can be detected by sequence studies or more commonly by serological means (i.e. by the use of antibodies directed to these differences).
1. IgG - γ - Gamma heavy chains
2. IgM - μ - Mu heavy chains
3. IgA - α - Alpha heavy chains
4. IgD - δ - Delta heavy chains
5. IgE - ε - Epsilon heavy chains
As adhesion-signaling molecules, immunoglobulins act as B-cell receptors (BCR) that recognize (ligate) specific antigens. The B cell receptor (BCR) is an integral membrane protein complex that is composed of two Ig heavy chains, two Ig light chains and two heterodimers of Ig-α and Ig-β. Antigens bind with greatest affinity to their cognate Ig-antibody, and hence to activated the BCR of greatest affinity.
Antigen-activated B cells proliferate and differentiate into memory B cells or plasma cells. B cell development is a tightly controlled process in which over 75% of the developing cells become apoptotic because of inappropriate immunoglobulin gene rearrangements or recognition of self antigens by Igs. Copious amounts of monoclonal antibodies are synthesized by antigen-stimulated B cell-derived plasma cells, while memory B cells remain primed for rapid activation by a repeat encounter with the initial, diffentiation activating antigen.
The enormous diversity of immunoglobulin molecules is attributable to VDJ recombination, while secondary antibody diversification is engineered by AID-induced/UNG2 assisted somatic hypermutation (SHM) or class-switch recombination (CSR), and AID-induced gene-conversion (GC). Affinity maturation is achieved are somatic hypermutation and clonal selection, which together ensure that repeated exposures to the same antigen will provoke greater antibody ligating affinity in the antibody secreted by successive generations of plasma cells.
tags [Proteins] [immunoglobulin] [isotypes] [diversity]

 Immunoglobulins (left - click to enlarge) comprise two heavy (H) and two light-chain (L) protein subunits, each of which folds into domains (4 on heavy, 2 on light). These adhesion sites or domains contain one or more folds of 60 to 100 amino acids.
Immunoglobulins (left - click to enlarge) comprise two heavy (H) and two light-chain (L) protein subunits, each of which folds into domains (4 on heavy, 2 on light). These adhesion sites or domains contain one or more folds of 60 to 100 amino acids.Depending upon the character of the heavy chain, immunoglobulins are divided into five classes – IgG, IgD, IgE, IgA, IgM – that are expressed in different tissues (detail). The classes are further subdivided into isotypes, which have different properties in terms of complement fixation and binding affinity to immunoglobulin (Ig) receptors.
Immunoglobulins can be divided into five different classes, based on differences in the amino acid sequences in the constant region of the heavy chains. All immunoglobulins within a given class will have very similar heavy chain constant regions. These differences can be detected by sequence studies or more commonly by serological means (i.e. by the use of antibodies directed to these differences).
1. IgG - γ - Gamma heavy chains
2. IgM - μ - Mu heavy chains
3. IgA - α - Alpha heavy chains
4. IgD - δ - Delta heavy chains
5. IgE - ε - Epsilon heavy chains
As adhesion-signaling molecules, immunoglobulins act as B-cell receptors (BCR) that recognize (ligate) specific antigens. The B cell receptor (BCR) is an integral membrane protein complex that is composed of two Ig heavy chains, two Ig light chains and two heterodimers of Ig-α and Ig-β. Antigens bind with greatest affinity to their cognate Ig-antibody, and hence to activated the BCR of greatest affinity.
Antigen-activated B cells proliferate and differentiate into memory B cells or plasma cells. B cell development is a tightly controlled process in which over 75% of the developing cells become apoptotic because of inappropriate immunoglobulin gene rearrangements or recognition of self antigens by Igs. Copious amounts of monoclonal antibodies are synthesized by antigen-stimulated B cell-derived plasma cells, while memory B cells remain primed for rapid activation by a repeat encounter with the initial, diffentiation activating antigen.
The enormous diversity of immunoglobulin molecules is attributable to VDJ recombination, while secondary antibody diversification is engineered by AID-induced/UNG2 assisted somatic hypermutation (SHM) or class-switch recombination (CSR), and AID-induced gene-conversion (GC). Affinity maturation is achieved are somatic hypermutation and clonal selection, which together ensure that repeated exposures to the same antigen will provoke greater antibody ligating affinity in the antibody secreted by successive generations of plasma cells.
tags [Proteins] [immunoglobulin] [isotypes] [diversity]
Labels: adhesion, affinity maturation, BCR, CSR, glycoproteins, immune, immunoglobulins, signaling







































